PLA Filament Featuring Cuprionix®, a Patented Copper Oxide Nanomposite
In October, 2020 the Pasteur Institute in Lille, France tested Copper3D’s Cuprionix®, the active additive ingredient in PLACTIVE AN1, against Human Coronavirus strain 229E (HCoV-229E), under the EN14476 + A2 standard, with extraordinary results. After contact at 20°C with human coronavirus HCoV-229E in clean conditions according to the method described in standard NF EN 14476+A2 (July 2019)*, product COPPER 3D Additive at 50 % shows virus copy reduction greater than or equal to 2.8 log after 30 seconds corresponding to a 99.842 % reduction in viral load.

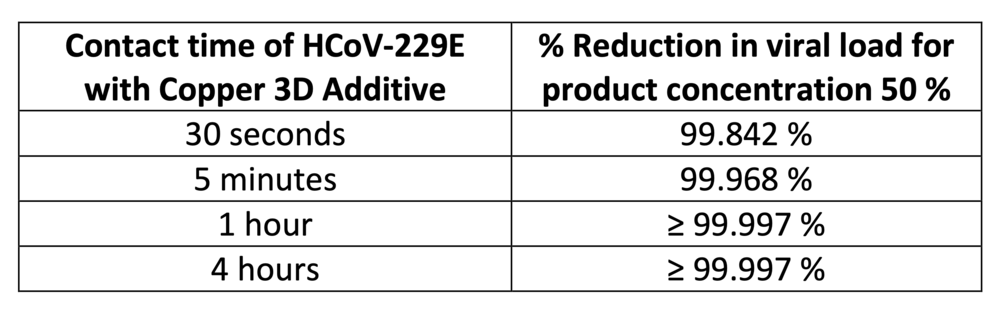
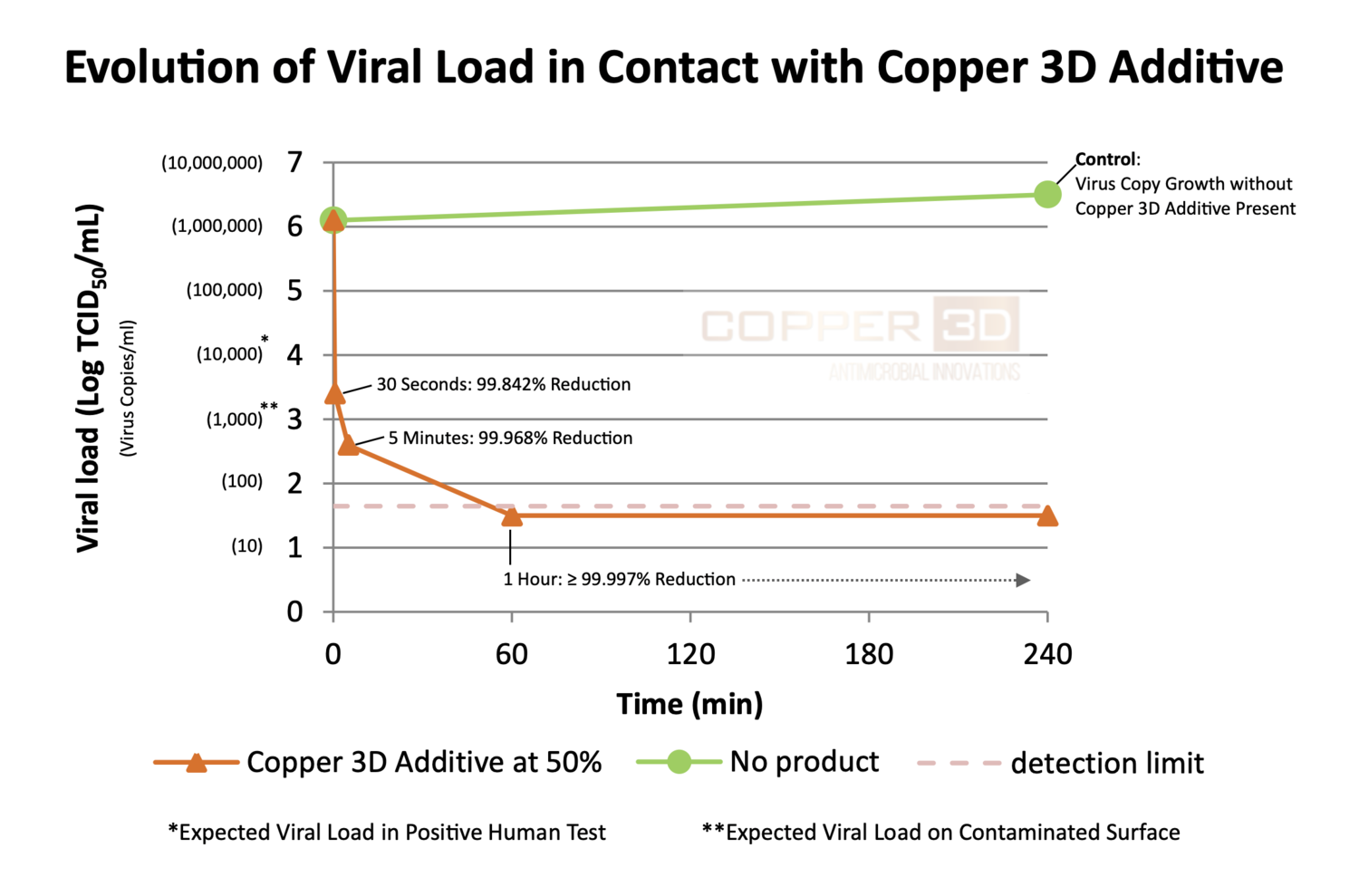
This test was conducted with an initial viral load of 1,000,000/ml (Log 6), which is roughly 1,000 times the expected viral load on a contaminated surface (Log 3), vs. the Copper3D Cuprionix® additive at at 50% concentration. Cuprionix® is the active additive ingredient in PLACTIVE AN1.
In addition Cuprionix® did not show any degree of cytotoxicity, indicating that it is also safe for use in applications that will be in contact with patients or animals.
FDA-compliant and ISO-certified, PLACTIVE AN1™ is an innovative Nanocomposite developed with a high quality PLA and a patented, scientifically validated and highly effective Nano-Copper additive.
For more information please contact us at: [email protected]
SHOP COPPER3D PLACTIVE AN1 HERE
IDEAL FOR 3D PRINTING FACE MASKS, DOOR OPENERS, & PHONE CASES
Patented and proprietary nano copper additive releases highly active copper ions.
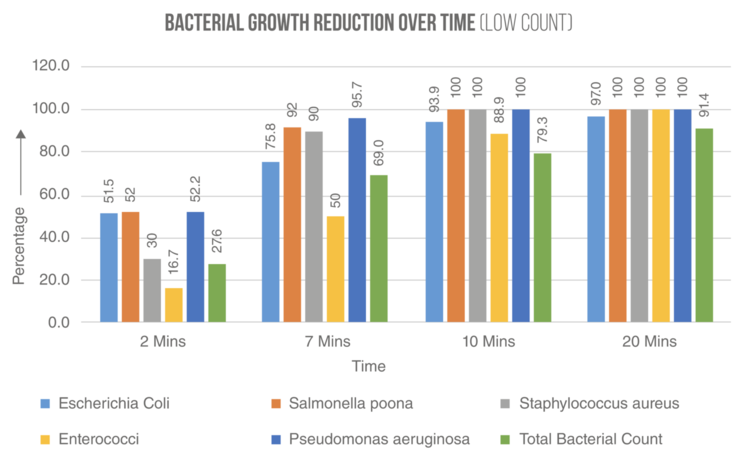
PLACTIVE ™ COPPER OXIDE ACTIVITY
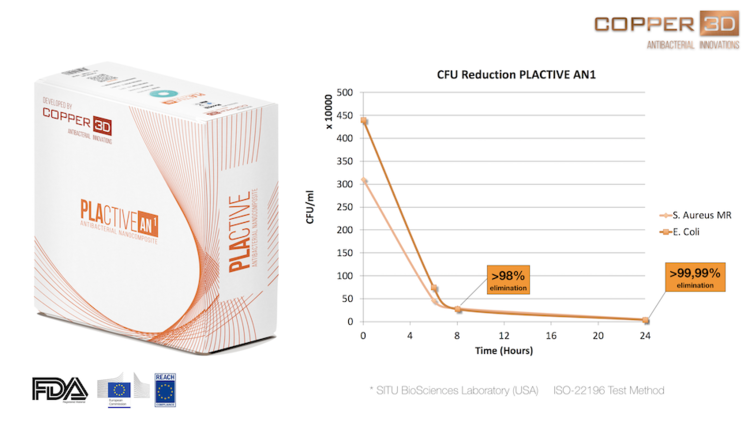
These graphics shows the results of studies conducted by microbiology laboratories in USA, Chile and Dubai1,2,3. These studies confirm that the Colony Forming Units (CFU) of Staphylococcus aureus MRSA and Escherichia coli DH5α and other common microbes, falls abruptly during the first minutes in contact with PLACTIVE, continuing the elimination of bacterial strains until reaching >99.99% elimination at 24 hours.
1. SITU Biosciences Microbiology Laboratory, USA.
2. Microbiology Laboratory of Universidad Católica de Valparaíso, Chile.
3. Al Hoty Stanger Laboratories, Dubai
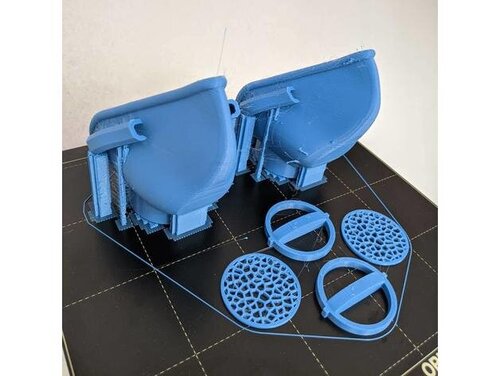
Ideal for 3d printing reusable face masks, door openers, cell phone cases, door knob covers, etc.


#HackThePandemic
Hospitals around the world are close to running out of N95 masks in the middle of the worst pandemic of our lifetimes. Our friends at Copper3D have developed an open source response to the COVID-19 pandemic that utilizes distributed manufacturing in conjunction with their groundbreaking copper oxide infused material..
There is a critical breakdown in the global stock of N95 masks, and they are a basic necessity, especially for Healthcare personnel who are at the forefront of fighting this disease. In many countries the authorities have also recommended the use of these masks (or similar) on public transport. These masks, despite being effective, also have some problems such as a short lifecycle (about 8 hours), and have another even more serious problem. Respiratory viruses, specifically SARS-Cov-2 (COVID-19) can live up to 72 hours on different surfaces. This is a problem since using a conventional mask, at the end of the day we would have a high viral/bacterial load trapped within millimeters of our nose and mouth, further exposing ourselves to these dangerous microbes.
Copper3D’s quest to alleviate this shortage has led to a low-cost, quick, and decentralized solution through distributed manufacturing.
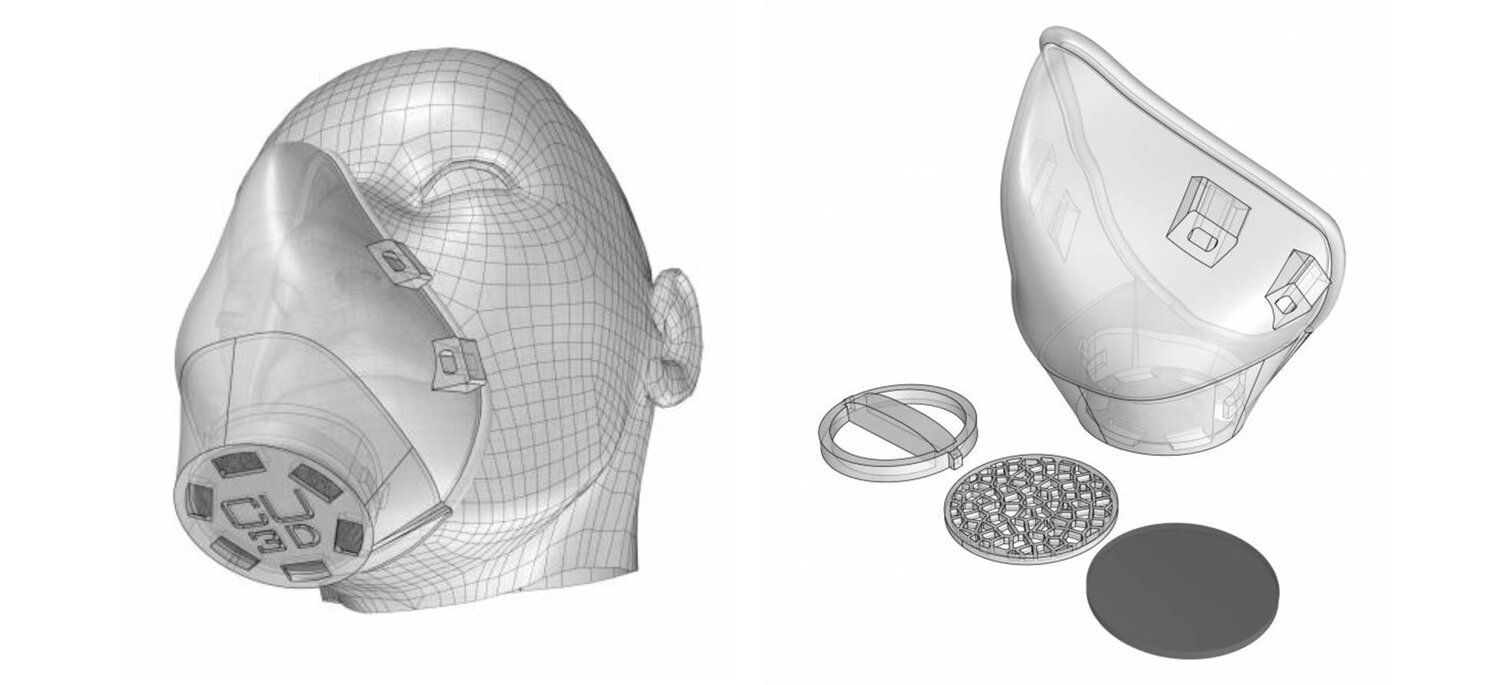
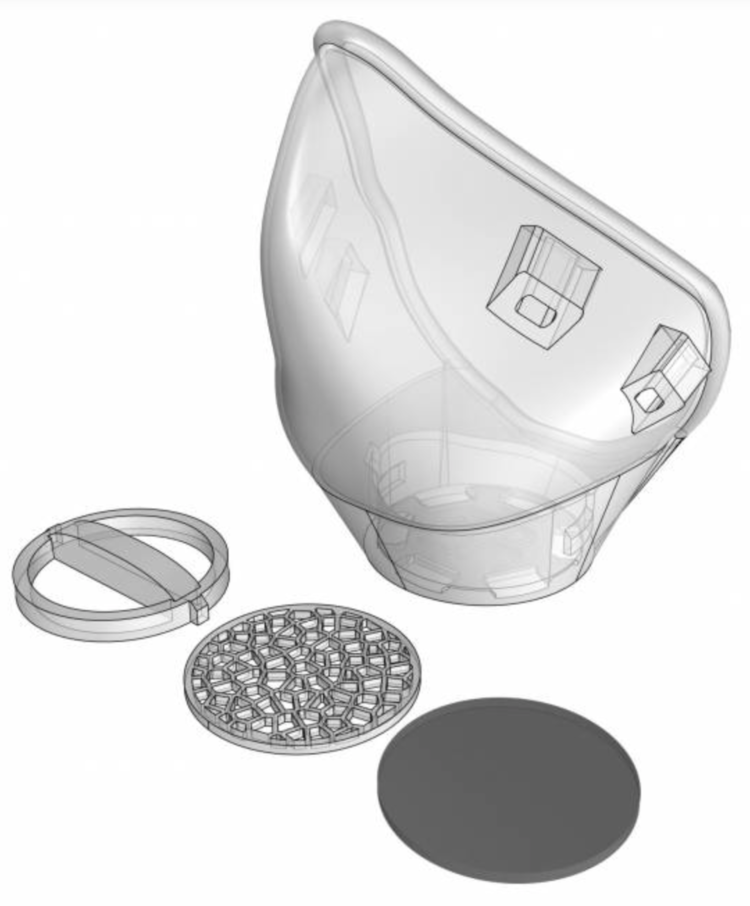
They have created a reusable, customizable, modular, copper oxide infused PLA 3d printed respirator system made with PLACTIVE AN1™. A nano-composite, FDA-compliant, and ISO-certified PLA filament.
Enter NANOHACK 2.2: THE OPEN SOURCE RESPIRATOR MASK
THIS IS LAST RESORT DEVICE, NOT INTENDED TO BE A REGULAR PPE (PERSONAL PROTECTIVE EQUIPMENT) OR A N95 MASK. AS ANY 3D PRINTED DEVICE, YOU SHOULD POST-PROCESS, CLEAN, AND SEAL THE MASK IN ORDER TO USE PROPERLY. WE DON’T RECOMMEND TO PRINT FACE MASKS WITH REGULAR PLA, PET-G OR TPU MATERIALS. THIS IS AN OPEN SOURCE FILE AND WE EXPECT YOUR COLLABORATION TO IMPROVE THIS DESIGN. - Copper3D
Click here to download the free STL packet for the Copper3D NanoHack respirator mask
Creative Commons License: “Attribution-NonCommercial 4.0 International (CC BY-NC 4.0)”
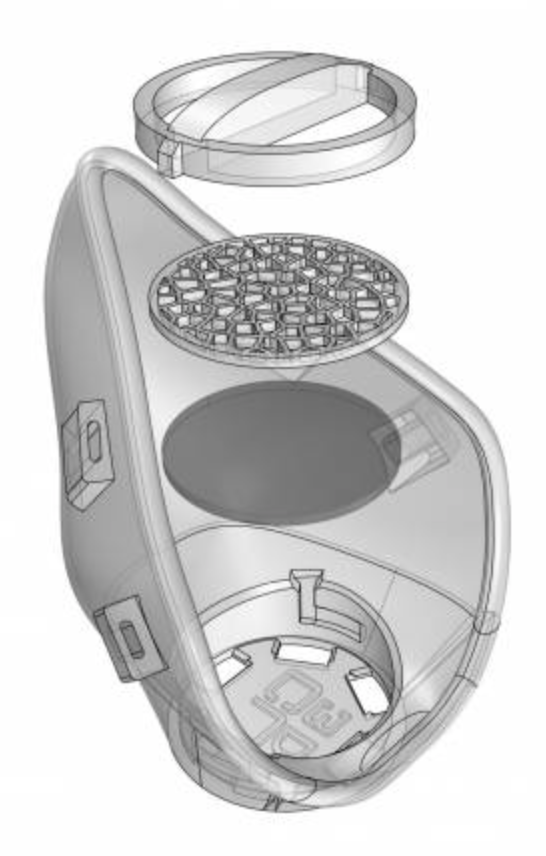
COPPER OXIDE INFUSED
Manufactured with PLACTIVE® an innovative Nanocomposite developed with a high quality PLA and a patented, scientifically validated and highly effective Nano-Copper additive.
MONOBLOCK STRUCTURE
NanoHack 2.2 is made of a monoblock PLACTIVE® structure to provide maximum protection against the external environment. Contrary to other concepts, NanoHack 2.2 is designed to be a strong and hermetic structure, sealed with a rim of Mdflex, a copper oxide infused TPU.
REUSABLE & RECYCLABLE
You can use this mask any times you want. The increasing use of single-used surgical masks and N95 respirators will have a detrimental effect in the ecosystem. To prevent this detrimental effect in our environment, NanoHack will be made with recyclable material.
MODULAR FILTRATION SYSTEM
NanoHack incorporate a novel modular filtration system manufactured with a copper nanocomposite polymer. This novel active filtration system also includes 3 layers of non-woven propylene embedded in nano-copper, and can house third party filtration materials.
TECHNICAL CONSIDERATIONS
NanoHack was conceived as an open source 3D printed face mask manufactured with active materials. These are some of the technical considerations prior to print NanoHack
ABOUT THE ACTIVE ACTIVITY OF COPPER
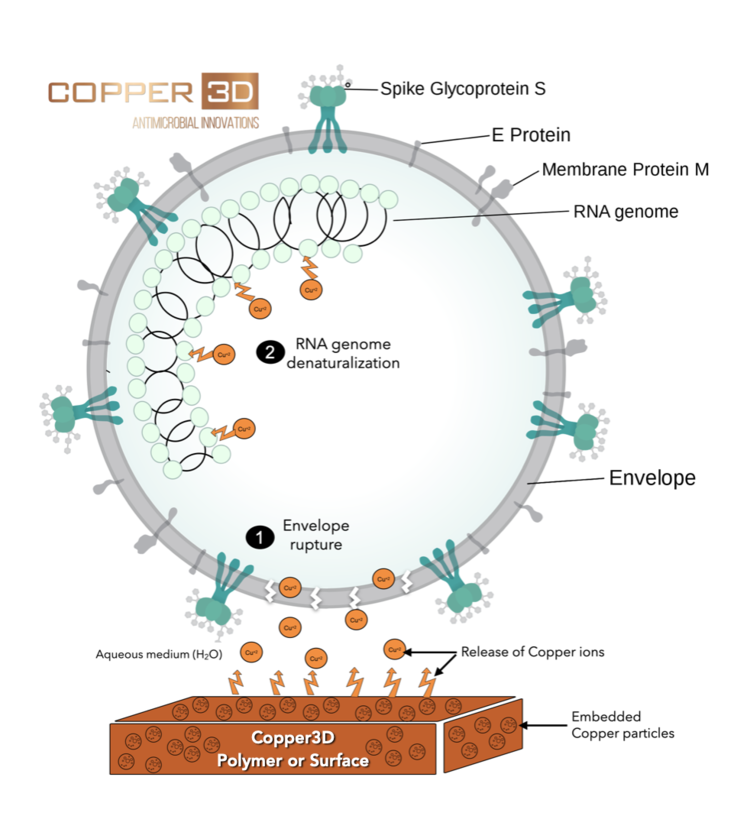
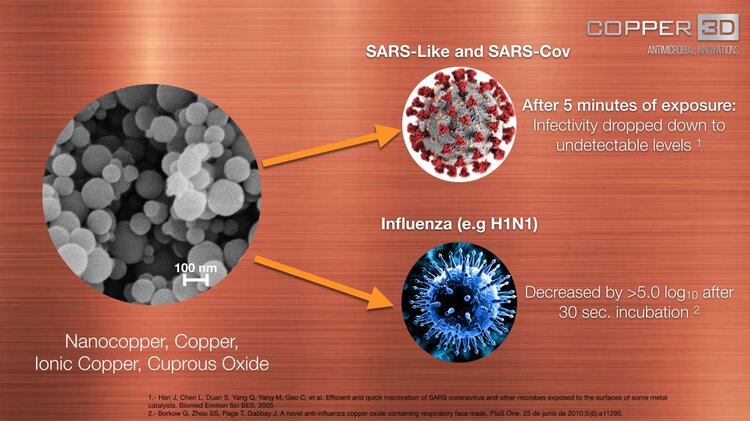
Copper and Nano Copper inhibits the replication and propagation abilities of SARS- CoV4, influenza5 and other respiratory viruses, Copper can inactivate viruses as SARS-like and SARS- Cov4, influenza virus5, H1N1, and eliminates dangerous bacteria like Staphylococcus aureus, Escherichia coli, Listeria, among others6, after a short period of exposure.
References
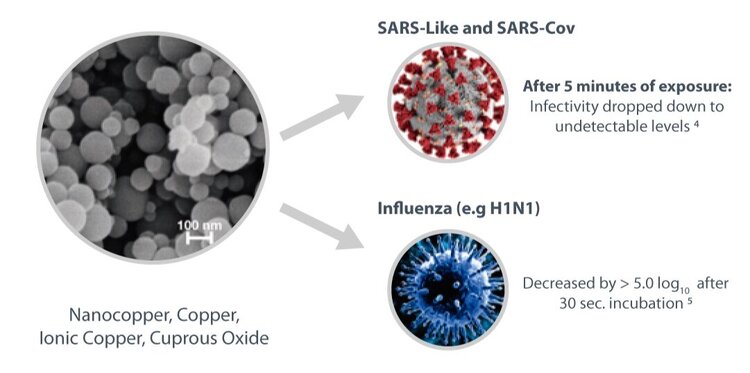
4. Han J, Chen L, Duan S, Yang Q, Yang M, Gao C, et al. Efficient and quick inactivation of SARS, coronavirus and other microbes exposed to the surfaces of some metal catalysts. Biomed Environ Sci BES, 2005.
5. Borkow G, Zhou SS, Page T, Gabbay J. A novel anti-influenza copper oxide containing respiratory face mask. PloS One. June 25, 2010;5(6):e11295.
6. Copper3D Inc. laboratory tests.
In order to achieve optimum protection against the external environment, we recommend using a triple-layer non-woven polypropylene filter embedded in nano-copper developed by The Copper Company.
If you do not have access to this type of filter, the study by Anna Davies et al1 analyze the filtering efficiency of different materials. See attached table:
ABOUT THE FILTRATION SYSTEM OF NANOHACK
NanoHack was conceived as a 3D printed face mask manufactured with active copper oxide infused materials. We will use active filters of non-woven polypropylene (3 layers) embedded in nanocopper to get extra protection.
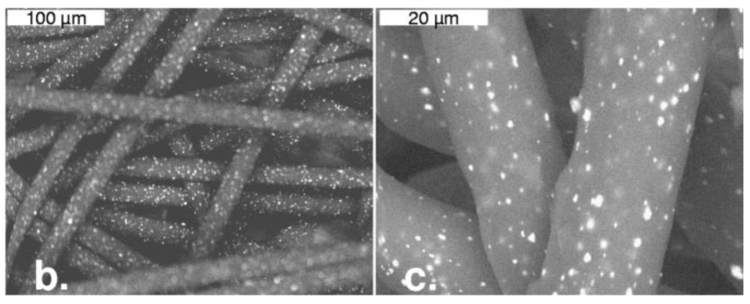
In the study by Borkow et al (2007)7, a 2.5 cm filter was designed containing a 2 cm thick top layer of 500 mg of non-woven polypropylen impregnated with 5% copper oxide particles. This study had a control that was non-woven polypropylen copper-free as a control.
Diffusion of viruses through filters containing copper oxide resulted in a significant reduction in viral titers from 0.47 log10 to 4.6 log10 depending on the virus analyzed.
References
7. Borkow G, etal., (2007) Neutralizing Viruses in Suspensions by Copper Oxide-Based Filters. Antimicrobial Agents and Chemotherapy, p. 2605–2607.
CLAIMS ABOUT NANOHACK:
The purpose of the NanoMask is to offer the general population a degree of protection against airborne particles and to prevent the spread of liquid aerosols that could contaminate the airways.
-
It is not an N95 mask. It is a face mask and should not be considered as a PPE.
-
If you are a healthcare professional, you should use it as a last resort device: you cannot manipulate airway such as intubation, mechanical ventilation, fiberoptic bronchoscopy and similar procedures.
-
You can use it in common spaces.
-
It should be used for a maximum of 8 hours and change the non-woven filter once a day. After handling the active filter, you should wash your hands and follow precautions as recommended by the health authority.
-
Nanohack is a polymer based device, we recommend using PLACTIVE® (PLA based material) for the monoblock structure and MDflex® (TPU based material) to print the outer rim. With this setup, you can have a comfortable mask with a good seal.
-
We understand that there may eventually be complex access to MDflex® in some parts of the world. This is why we have released a version with an integrated rim into the monoblock structure. With this solution you can obtain a structure that fits properly but that must be complemented with some additional sealant and hypoallergenic cushioned tapes for nose and cheeks, especially if it is going to be used for long periods of time.
-
Keep in mind also that if you leave the elastic bands very tight it can hurt you, we don’t want the use of NanoHack to be counterproductive or cause you discomfort.
-
The NanoHack was designed to fit a 12 cm height face very well, measured from the tip of the chin to the ocular plane, a horizontal line that passes just between the eyes, and a distance between cheekbones (measured straight above the nose) also 12 cm.
-
We understand that all faces are different. If your face is smaller or larger than these measurements, we suggest rescaling the model by 5% or 10% so that there is a perfect fit to your face.
CLEANING CONSIDERATIONS
1. Washing: Wash the equipment with soap (e.g. liquid dish soap) and clean water.
2. Rinsing: Rinse the equipment completely with clean water.
3. Disinfect: Disinfect the equipment to inactivate any remaining pathogens. Use chemical disinfection if plastic part cannot tolerate 80°C. Different countries have different disinfection protocols. Here are the most accessible chemical germicides and methods:
- 3.1. Method 1: Alcohol is effective against influenza virus. Ethyl alcohol (70%) is a powerful broad-spectrum
germicide and is considered generally superior to isopropyl alcohol. Since alcohol is flammable, limit its use as a surface disinfectant to small surface-areas and use it in well-ventilated spaces only. Prolonged and repeated use of alcohol as a disinfectant can also cause discoloration, swelling, hardening and cracking of rubber and certain plastics.
- 3.2. Method 2: Most household bleach solutions contain 5% sodium hypochlorite (50, 000 parts per million available chlorine). Recommended dilution: 1:100 dilution of 5% sodium hypochlorite is the usual recommendation. Use 1-part bleach to 99 parts cold tap water (1:100 dilution) for disinfection of surfaces. Adjust ratio of bleach to water as needed to achieve appropriate concentration of sodium hypochlorite. For example, for bleach preparations containing 2.5% sodium hypochlorite, use twice as much bleach (i.e. 2 parts bleach to 98 parts water).
4. Rinsing: If using chemical disinfection, rinse with sterile or clean water (i.e. water boiled for 5 minutes and cooled). Sterile water is preferred for rinsing off residual liquid chemical disinfectant from a respiratory device that has been chemically disinfected for reuse, because tap or distilled water may harbor microorganisms that can cause pneumonia. However, when rinsing with sterile water is not feasible, instead, rinse with tap water or filtered water (i.e. water passed through a 0.2 μ filter). Disinfection by immersion is recommended with a contact time of 30 minutes.
5. Dry equipment: Follow the previous step by an alcohol rinse and forced-air drying.
6. Store: Store equipment dry in closed packages.
1.Infection Prevention and Control of Epidemic- and Pandemic-Prone Acute Respiratory Infections in Health Care. Geneva: World Health Organization; 2014. Annex I, Cleaning and disinfection of respiratory equipment. Available from: https://www.ncbi.nlm.nih.gov/books/NBK214361
NanoHack Mask is a last resort device with the purpose of offering protection from airborne particles and prevent spreading liquid contaminating the airways. Published data1 has shown that the filtration materials used by NanoHack (non-woven polypropylene, the same material used in surgical masks) achieves a filtration efficiency of 96.4% for microorganisms of 1 micron and 89.5% for microorganisms of 0.02 microns1.
According to the U.S. Food and Drug Administration (FDA), the design of surgical masks do not allow a complete protection from germs and other contaminants due to their loose fit2. In addition, surgical masks are single-used devices required to be safely disposed. The Centers for Disease Control and Prevention (CDC) recommends placing these items it in a plastic bag and put it in the trash, then wash your hands after handling the used mask2. Previous published research3 has indicated that the high viral load remaining in surgical masks and respirators, can be a source of viral transmission both to the person wearing the mask or respirators and to others3. This may happen when healthcare workers touch their mask and then fail to wash their hands properly or when they dispose of the mask without proper safe disposal precautions3. In addition, pathogens shedding from surgical respirators onto patients in the operating room, increasing the risk of nosocomial infections3. Thus, NanoHack Mask uses a recyclable and biocompatible polymer containing a copper nanocomposite.
References
1.Anna Davies, et al. Testing the Efficacy of Homemade Masks: Would They Protect in an Influenza Pandemic? Disaster Medicine and Public Health Preparedness, Available on CJO 2013 doi:10.1017/dmp.2013.43.
2.Food and Drug Administration (FDA): https://www.fda.gov/medical-devices/personal-protective-equipment-infection-control/n95-respirators-and-surgical-masks-face-masks. Accessed March 20, 2020.
3.Borkow G, etal., (2010) A Novel Anti-Influenza Copper Oxide Containing Respiratory Face Mask. PLoS ONE 5(6): e11295. doi:10.1371/journal.pone.0011295.
PLACTIVE AN1™ Copper Oxide Nanocomposite Infused PLA Filament
· 10% of all worldwide patients admitted in a hospital will acquire at least one healthcare-associated infection (HAI)1· Annually in the US, 2 million of patients gets infected in hospitals2· 90.000 of them die2
· The estimated cost for the US Healthcare System is up to 45 Billion USD2, 3
· 50% of HAI’s at worldwide level are attributable to bacterial contamination of Medical Devices4
· >40% of Amputees present dermic complication due to High Bacterial Burden of Prosthetics and Orthotics5
PLACTIVE AN1(tm) is an innovative Nanocomposite developed with a high quality PLA and a patented, scientifically validated and highly effective Nano-Copper additive. This unique combination of technologies brings the following characteristics to Copper3D products:
- Copper oxide nanoparticles infuse PLACTIVE AN1 with the special properties of copper. Copper has been shown to have protective properties.
- Copper oxide infused properties confirmed by two microbiology laboratories in Chile and USA.
- Clinically tested in prosthesis for amputees with excellent results.
PLACTIVE AN1(tm) is ISO 10993-10: 2010 certified for biocompatibility of Skin Contact/Mucose Contact.
PLACTIVE(tm) is a FDA Registered Material and EU compliant (No. 10/2011, No. 1935/2004 and No. 2023/2006). The manufacturer also has ISO 9001/2015 certification and is REACH compliant.
Contains copper oxide. No human health claims are made.
PLACTIVE(tm) Applications
PLACTIVE AN1 is ideal for the manufacturing of medical applications where it's dangerous to have bacterial contamination, such as postoperative prostheses, wound dressing and surgical equipment.
Copper oxide nanoparticles infuse PLACTIVE AN1 with the special properties of copper. Copper has been shown to have protective properties
Copper oxide infused properties confirmed by two microbiology laboratories in Chile and USA.
PLACTIVE™ is a FDA Registered Material and EU compliant (No. 10/2011, No. 1935/2004 and No. 2023/2006).
The manufacturer also has certification ISO 9001/2015 and is REACH compliant.

Clinically tested in prosthesis for amputees with excellent results. Ideal for the manufacture of other medical applications where it’s dangerous to have bacterial contamination, such as postoperative prostheses, wound dressing and surgical equipment.
The Nano-Additive mantains all the mechanical properties of the base PLA material.
PLACTIVE™ is currently being tested by NASA to evaluate its impact on interplanetary microbial contamination.
Non-toxic product and environmentally friendly (biodegradable).
See more information here: Copper3D
Copper3D (www.copper3d.com) is a chilean company born in an academic process in the Master of Innovation at the Adolfo Ibañez University, the most important business school in LatAm. In this academic program, the founding team of this company met:

Andrés Acuña, Electronic Civil Engineer with 14 years of experience in the mining sector and specializations in automation of mineral processing.
Daniel Martínez, Physical Therapist & MBA, with 13 years of experience in management, marketing, innovation and new business models in the Healthcare industry.
Claudio Soto MD, Physician with vast experience in rehabilitation, regulatory aspects of Healthcare and process management in large health institutions.
We realized that more than 40% of amputees suffer some type of dermic disorders due to the use of their prostheses. This

phenomenon is also observed in non-amputee patients who use orthoses. The reason? The high bacterial burden present in these medical devices, which in contact with the skin can cause dermatitis, folliculitis, fungal or bacterial infections, among others.

But then we realized that the problem of bacterial burden is much more complex and huge: According to some studies, about 50% of all HAI’s (Hospital Acquired Infections) worldwide are due to the bacterial burden of medical devices that are difficult to maintain clean and sterilyzed.
After a long process of research, development and innovation, we realized that the ideal solution to this problem involved the molecular intervention of the
material with which these medical devices are manufactured. After discarding many options, we began prototyping a new polymer for 3D printing with an internationally patented additive containing copper nanoparticles, extremely effective in eliminating fungi, viruses and bacteria but safe for humans at the right concentrations.
With successful preliminary trials in Chile, we decided to start with the industrial manufacture of this material in Europe for later commercialization.
At the moment we have our first product available, it is a high quality PLA polymer with additive concentrations of 1 and 2%, it is called PLACTIVE since it is a PLA polymer with “active" properties in the elimination of a wide range of microorganisms.
We also developed a medical grade material called NANOCLEAN, it is a high quality PETG polymer with additive concentrations of 2 and 3% and aimed at more specific purposes in the world of medical devices.
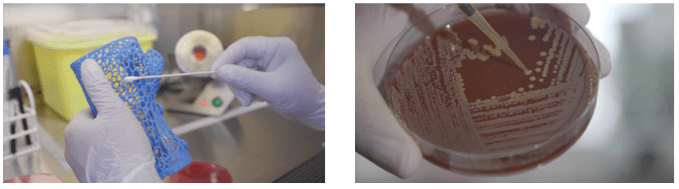
CLINICAL USE Nº10:
The Otoscope is one of the most used Medical Devices for its simplicity and versatility when it comes to examining ears, nose and throat.
But it has a weak point. The cone of the otoscope is the piece that comes into direct contact with the ear. It is a plastic piece (hence porous) that accumulates a high bacterial load if it is not cleaned or disposed of properly. This often causes patients (usually children) examined with contaminated otoscopes to leave the clinic / hospital with an otitis that falls within the group of Infections Associated with Health Care (IAAS).
You can find detailed instructions for its construction here, even with its lighting system:
https://www.wevolver.com/…/fie…/blob/master/documentation.md
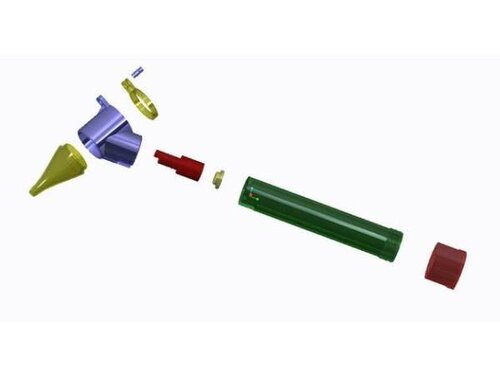
CLINICAL USE Nº11:
3D PRINTED SURGICAL SEPARATOR Surgical separators are key pieces in any operating room in the world. Historically, they have been manufactured in stainless steel, but since it is a “passive” material, it allows to colonize quickly in the presence of even a minimal amount of microorganisms. A study published in the Journal of Surgical Research by the department of surgery of the U. of Arizona: (https://lnkd.in/dd_VNwA) concluded that these pieces can be perfectly manufactured with 3D printing, they take about 90 minutes to built, the final piece easily supports the forces necessary to fulfill its function within an operating room (+ 13Kg of Tangential Force) and only at ONE TENTH of the cost of the original piece of steel. If in addition to all these attributes, we add copper oxide infused and REUSABLE if manufactured with PLACTIVE OR NANOCLEAN (www.copper3d.com), so we have a new class of surgical, functional, cost-effective, in-situ manufacturing, and customizable, reusable separators that are manufactured with an ACTIVE material.
CLINICAL USE Nº 12

3D PRINTED SURGICAL SEPARATOR Surgical separators are key pieces in any operating room in the world. Historically, they have been manufactured in stainless steel, but since it is a “passive” material, it allows to colonize quickly in the presence of even a minimal amount of microorganisms. A study published in the Journal of Surgical Research by the department of surgery of the U. of Arizona: (https://lnkd.in/dd_VNwA) concluded that these pieces can be perfectly manufactured with 3D printing, they take about 90 minutes to built, the final piece easily supports the forces necessary to fulfill its function within an operating room (+ 13Kg of Tangential Force) and only at ONE TENTH of the cost of the original piece of steel. If in addition to all these attributes, we add copper oxide infused and REUSABLE if manufactured with PLACTIVE OR NANOCLEAN (www.copper3d.com), so we have a new class of surgical, functional, cost-effective, in-situ manufacturing, customizable, reusable separators , manufactured with an ACTIVE material.